electron configuration for iodine|Electron Configuration Chart of All Elements (Full Chart) : Pilipinas Mar 23, 2023 We would like to show you a description here but the site won’t allow us.
PH0 · Iodine, electron configuration
PH1 · Iodine – Electron Configuration and Oxidation States – I
PH2 · Iodine Electron Configuration: Everything You Need to Know
PH3 · Iodine Electron Configuration (I) with Orbital Diagram
PH4 · Iodine (I)
PH5 · Iodine
PH6 · Electron configuration for Iodine (element 53). Orbital diagram
PH7 · Electron Configuration Chart of All Elements (Full Chart)
PH8 · Complete Electron Configuration for Iodine (I, I– ion)
PH9 · A step
With backgammon HTML5 web apps ('blue app' & 'green app'), you can play backgammon using any new browser - either on a desktop like Windows or Mac, or on a mobile device like iPhone or iPad or Android phone or tablet. For best experience with GameColony web apps, we recommend using Google Chrome web browser. You can .
electron configuration for iodine*******Electron configuration for Iodine (element 53). Orbital diagramIodine Electron Configuration: Everything You Need to Know
Electron Configuration Chart of All Elements (Full Chart)Iodine – Electron Configuration and Oxidation States – IComplete Electron Configuration for Iodine (I, I– ion)
electron configuration for iodine Mar 23, 2023
electron configuration for iodine Electron Configuration Chart of All Elements (Full Chart) In order to write the I electron configuration we first need to know the number of electrons for the I atom (there are 53 electrons). When we write the configuration, we'll put all 53. Iodine Number of Valence Electrons. Iodine has seven Valence Electrons. What is The Electron Configuration of Iodine. There are 53 electrons in iodine that occupy the respective orbitals as given below; 1s² 2s² .Iodine electron configuration. ← Electronic configurations of elements. I (Iodine) is an element with position number 53 in the periodic table. Located in the V period. Melting point: 113.5 ℃. .
Iodine is a nonmetal with atomic number 53 and symbol I. Its electron configuration is [Kr] 4d 10 5s 2 5p 5, with valence electrons of 7 and valency electrons of 1,3,5,7.
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. Boiling point .Iodine. Full electron configuration of iodine: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 5. tellurium ← iodine → xenon Electron configuration of Iodine is [Kr] 4d10 5s2 5p5. Possible oxidation states are +1,5,7/-1. Electron Configuration. The periodic table is a tabular display of the chemical . Let us discuss the energy levels that enclose an atom. The electronic configuration of [I] is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4d10 5s2 5p5, with atomic number 53. Iodine (I) is .The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells . How does the electronic configuration of Iodine explain its chemical properties? Iodine has 7 valence electrons, which makes it highly reactive and prone to forming chemical bonds with other elements in order to complete its octet. This reactivity allows Iodine to form a wide range of compounds with other elements, including metals and non-metals.
The Electron configuration of iodine is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s²4d¹⁰ 5p⁵. Iodine, also called iodine is defined as the chemical element that belongs to the periodic table. It is located in group 17, more precisely in the halogens, its atomic number is .
Electrons and Electron Configuration. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Iodine is 53. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative .
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait — you can avoid all this if you use our .
The iodine orbital diagram is a graphical representation of the electron configuration of the iodine atom. This diagram shows how the electrons in the iodine atom are arranged in different orbitals.
Configuration. σ g 2 π u 3 π g 3 σ u 2. 7 . of the D state excited by the 1830 atomic line of iodine. The system further includes the diffuse emission bands in the region 2500 - 5000 with a . Schwarz, W.H.E., Inner electron excitation of iodine in the gaseous and solid phase, J. Chem. Phys., 1973, 58, 2230. Venkateswarlu, 1970 .This structure is called an electron configuration and is unique to hydrogen. Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells contain one s orbital which can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s 2 (spoken as “one-ess-two”).
In this video we will write the electron configuration for I- the Iodide ion. We’ll also look at why Iodine forms a 1- ion and how the electron configuration.Element Iodine (I), Group 17, Atomic Number 53, p-block, Mass 126.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs.
Excited state Iodine electron configuration. The first excited state electronic configuration of [I] is [Kr] 4d 10, 5s 2, 5p 4, and 5d 1, which is characterized by the transition of one electron from 5d to 6s orbital, creating 5d 8 and 6s 2 .

Iodine is the fourth halogen, being a member of group 17 in the periodic table, below fluorine, chlorine, and bromine; since astatine and tennessine are radioactive, iodine is the heaviest stable halogen. Iodine has an electron .
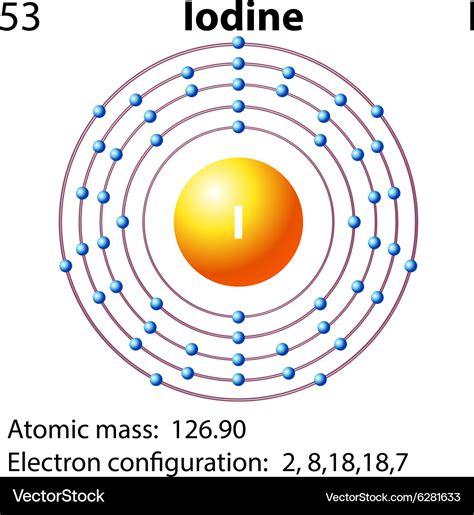
Iodine atoms have 53 electrons and the shell structure is 2.8.18.18.7. The ground state electron configuration of ground state gaseous neutral iodine is [Kr].4d 10.5s 2.5p 5 and the term symbol is 2 P 3/2.
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the .Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 5s 2 p 5; Electrons per Energy Level: 2,8,18,18,7 Shell Model; . Iodine - I (EnvironmentalChemistry.com)- Comprehensive information for the element Iodine - I is provided by this page including scores of properties, element names in many languages, most known nuclides and .Electronic configuration of the Iodine atom. Valence electrons. Orbital diagram. Iodine electron configuration. ← Electronic configurations of elements . I (Iodine) is an element with position number 53 in the periodic table. Located in the V period. Melting point: 113.5 ℃. Electron Configuration and Oxidation States of Iodine. Electron configuration of Iodine is [Kr] 4d10 5s2 5p5. Possible oxidation states are +1,5,7/-1. Electron Configuration. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties.Iodine atoms have 53 electrons and the shell structure is 2.8.18.18.7. The ground state electron configuration of ground state gaseous neutral iodine is [Kr].4d 10.5s 2.5p 5 and the term symbol is 2 P 3/2.
It’s often important to find an Electrophysiologist who has extensive experience treating your specific health condition. But it can also be challenging to find the best Electrophysiologist near Illinois, US. User review sites like Yelp are often of minimal help, especially since there can be a number of problems with relying on reviews of Electrophysiologists from other .Unfortunately, when I compile the code below, the program runs, but I receive a warning: "no such slot QObject::flip() in game.cpp". It seems that my slot is not properly defined. Using a Youtube tutorial about QTimer, it sounded as though I needed to add the "Q_OBJECT" macro inside the game class (this is commented out below).
electron configuration for iodine|Electron Configuration Chart of All Elements (Full Chart)